Abstract
Introduction: Chemotherapy-based lymphodepletion (LD) with fludarabine and cyclophosphamide is considered as a standard LD regimen for chimeric antigen T-cell (CART) therapy. The national shortage of fludarabine has caused disruption in the patient care of those in need of CART therapy and highlights the urgent need for an alternative LD regimen. Bendamustine (benda) is a purine analog and an alkylating chemotherapy that has been used as an LD agent for tisagenlecleucel (Ghilardi et al.,2022). However, there is no data for benda LD in other commonly used CART products for relapsed or refractory non-hodgkin lymphoma (rel/ref NHL).
Methods: In this single institution study we sought to analyze CART expansion, toxicity and clinical outcomes for patients receiving CART therapy with benda as a LD agent (90mg/m2 on days -4 and -3). Patients were prospectively enrolled for data collection and correlative studies. Patients with the following histology were included, large B cell lymphoma (LBCL), transformed follicular lymphoma (tFL), Richter's transformation (RT), and mantle cell lymphoma (MCL). Informed consent was obtained from all the patients. Response was assessed using the Lugano 2014 criteria and toxicities were assessed by the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 and, the American Society for Transplantation and Cellular Therapy (ASTCT) consensus grading. CD19 CART expansion was measured by real-time flowcytometry with anti-idiotype-FMC63 conjugated to Dylight 650 (target antigen FMC63ScFV, Clone 136.20.1).
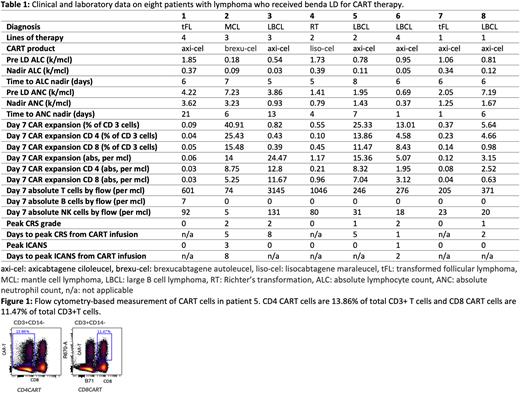
Results: In this ongoing study, 17 consecutively treated patients with lymphoma have received benda LD for standard of care CART. Median age of patients is 63 years, with majority of patients with a diagnosis of r/r LBCL (n=11 LBCL, n=4 tFL, n=1 RT, n=1 MCL). Eight patients are evaluable with day 7 CAR-T expansion data at the time of this report (Table 1). Median ALC nadir was 110/mcl (range 30-390) and median ANC nadir was 1340/mcl (range 370-3620). Only patient 6 developed grade 4 neutropenia with ANC nadir of 370/mcl; pre-LD ANC was 690/mcl. Median time to nadir ALC from first dose of benda was 6 days (day +1 relative to day 0 CART infusion) and median time to nadir ANC was also 6 days.
All patients demonstrated CART cell expansion detected by anti-idiotype immune phenotyping days 7, 14, 21, and 28 with median day 7 CAR expansion being 4.1/mcl (range 0.06-24.47/mcl). Figure 1 shows flowcytometry data on patient 5 as an example. Median day 7 CD4 CART expansion was 2.23/mcl and CD8 was 2.04/mcl. All but one patient had undetectable peripheral B lymphocytes at day 7 and day 14 demonstrating on-target effect of CART cells. Median peak CRS grade was 1 (with grade 2 CRS seen in 3 of the 8 patients) and ICANS was seen in two patients (grade 1 and grade 3). No unexpected side effects from benda LD were seen.
Conclusions: Benda LD is a safe and results in significant CART cell expansion sparing undesired neutropenia for CART therapy. We expect to accrue ~40 patients with NHL on this prospective clinical study with a priori plan to compare benda-LD with historical 150 fludarabine-cyclophosphamide-LD CART patients. Efficacy data, healthcare utilization, and translational CART PK data will be presented at the meeting.
Disclosures
Latchford:Kite Gilead: Speakers Bureau; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees. Arai:Kadmon: Membership on an entity's Board of Directors or advisory committees. Lowsky:Orca Bio: Research Funding. Negrin:CoImmune: Current equity holder in private company, Current holder of stock options in a privately-held company; University of Pennsylvania: Other: DSMB or Advisory Board; Novartis: Consultancy; UptoDate: Honoraria; Amgen: Consultancy; Kuur: Consultancy; Garuda: Consultancy; Magenta: Consultancy, Current equity holder in publicly-traded company; BioEclipse Therapeutics: Current equity holder in private company, Current holder of stock options in a privately-held company. Shizuru:Jasper Therapeutics: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; rBio: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Shoreline BioSciences: Current equity holder in private company, Honoraria, Membership on an entity's Board of Directors or advisory committees. Meyer:Orca Bio: Research Funding; indee labs: Membership on an entity's Board of Directors or advisory committees; Triursus Therapeutics: Other: Co-founder, scientific advisor; GigaGen: Other: Co-founder, scientific advisor. Shiraz:Kite, a Gilead company: Research Funding. Sidana:Oncopeptides: Consultancy; Sanofi: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding; Allogene: Research Funding; Janssen: Consultancy, Research Funding; Magenta Therapeutics: Consultancy, Research Funding; Prothena: Honoraria. Mackall:Immatics: Consultancy; Mammoth: Divested equity in a private or publicly-traded company in the past 24 months; Ensoma: Divested equity in a private or publicly-traded company in the past 24 months; Link: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months; Syncopation: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months; Nektar: Consultancy; Medimmune Tech: Consultancy; BMS: Consultancy; Apricity: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months; Lyell Pharmaceuticals: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months; GSK: Consultancy. Frank:Allogene Therapeutics: Research Funding; Roche/Genentech - Wife: Current equity holder in private company, Current holder of stock options in a privately-held company; Kite/Gilead: Honoraria, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Research Funding. Miklos:Kite, a Gilead Company: Research Funding; Allogene: Research Funding; Janssen: Consultancy, Honoraria; Bristol Meyers Squibb: Consultancy; Adaptive Biotech: Consultancy; Pharmacyclics: Patents & Royalties: cGVHD Ibrutinib patent ; Novartis: Consultancy; Fosun Kite: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal